Rocketvax and HALIX Collaborate to Advance the VIRUFLEX(TM) Platform and Next-Generation Nasal COVID-19 Vaccine
Introducing the VIRUFLEXTM Platform and HALIX’s Role
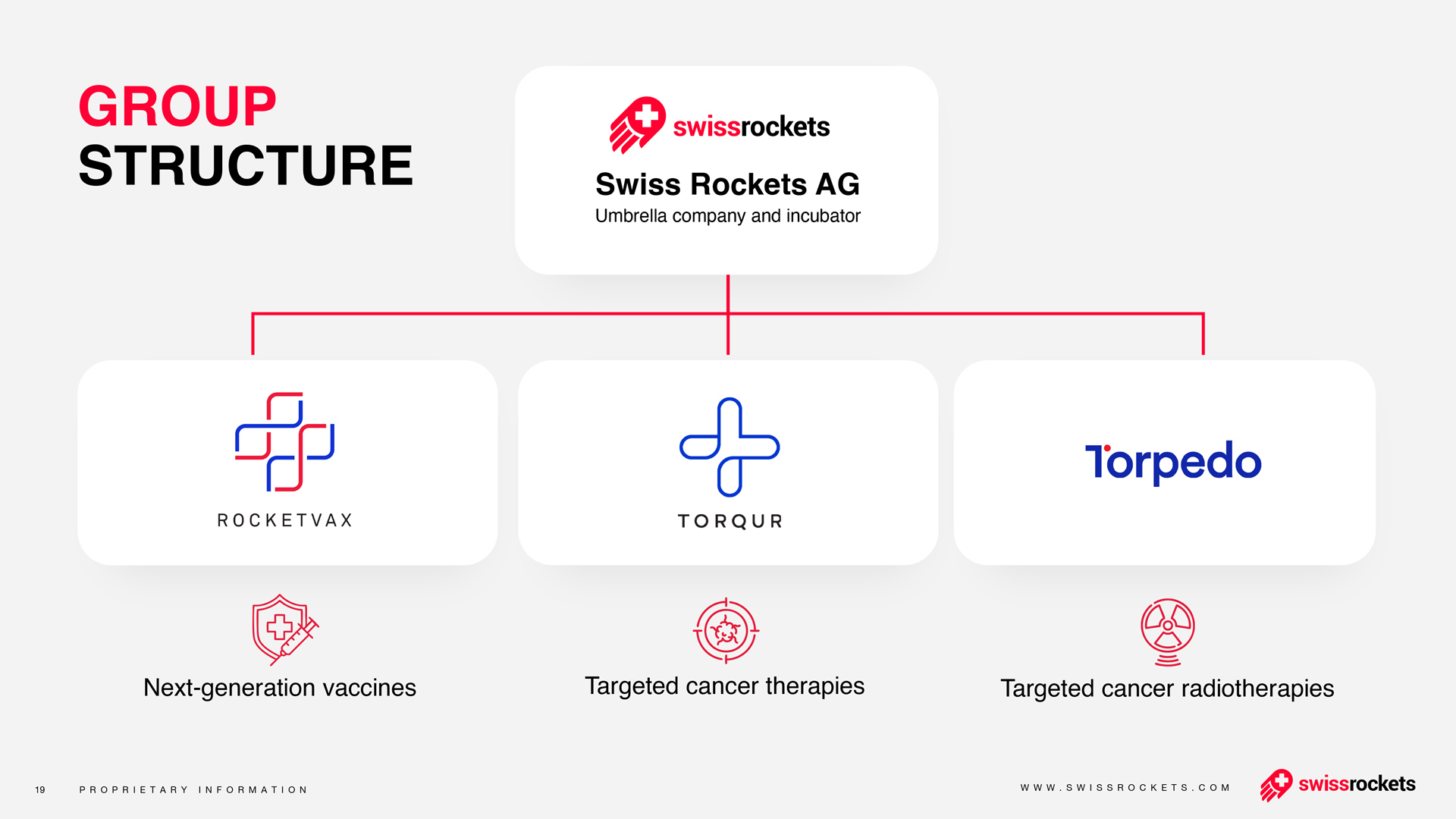
VIRUFLEXTM is Rocketvax’s state-of-the-art endemic readiness platform, designed to accelerate the development and deployment of next-generation vaccines in response to future pandemics. It integrates cutting-edge vaccine technology, scalable production processes, and global partnerships to ensure rapid responses to emerging infectious diseases.
HALIX has been selected as a strategic CDMO partner for VIRUFLEXTM, bringing world-class expertise in vaccine manufacturing to this pioneering initiative. HALIX’s contributions include:
• GMP-Grade Manufacturing: Ensuring the highest standards of quality and safety in vaccine production, adhering to rigorous Good Manufacturing Practices (GMP).
• Expertise in Viral Vectors: Leveraging specialized experience in viral vector manufacturing to enhance the efficiency and scalability of Rocketvax’s vaccine candidates.
• Scalability and Readiness: Offering flexible infrastructure to enable rapid scale-up of vaccine production, aligning with VIRUFLEX’s mission to ensure global vaccine availability during pandemics.
Advancing the Next-Generation Nasal COVID-19 Vaccine
Rocketvax’s innovative nasal COVID-19 vaccine represents a breakthrough in mucosal immunity and pandemic preparedness. This vaccine is a testament to Rocketvax’s proprietary SARS-CoV-2 "reverse genetic engineering" technology, which enables the development of a live-attenuated SARS-CoV-2 virus vaccine that offers protection against COVID-19 and potentially Long-COVID.
Key Differentiation Points:
1. Nasal Immunity: The nasal administration induces a strong localized immune response capable of neutralizing the wild-type virus at its entry point into the host, providing robust protection.
2. Broad Protein Recognition: Unlike conventional vaccines that target only the spike protein, Rocketvax’s vaccine trains the immune system to recognize all SARS-CoV-2 proteins, ensuring effectiveness against all virus variants.
3. Comprehensive Immune Activation: The vaccine activates the full spectrum of immune system components, including antibodies and immune cells. Animal studies demonstrate prolonged immune protection, a significant improvement over currently available mRNA vaccines.
4. Prevention of Virus Transmission: In contrast to mRNA vaccines, Rocketvax’s nasal vaccine prevents transmission of the wild-type virus, contributing to community-wide protection.
5. Room Temperature Stability: The vaccine’s stability at room temperature simplifies storage and distribution, eliminating the need for ultra-cold storage required by mRNA vaccines, thereby improving accessibility worldwide.
Partnership with the US Government
The development of Rocketvax’s nasal COVID-19 vaccine is further strengthened by its collaboration with the U.S. National Institutes of Health (NIH). This partnership aims to advance clinical development and expedite global access to this next-generation vaccine.
Statements from Leadership
“We are thrilled to partner with HALIX to bring the VIRUFLEXTM platform to life and revolutionize global pandemic readiness. With HALIX’s expertise in vaccine manufacturing, we are poised to deliver innovative solutions, including our groundbreaking nasal COVID-19 vaccine,” said Dr. Vladimir Cmiljanovic, CEO of Rocketvax AG.
“The VIRUFLEXTM platform exemplifies the innovative spirit needed to address global health challenges. We are honored to support Rocketvax in this mission, leveraging our manufacturing expertise to ensure the success of their vaccine candidates,” said Dr. Lutz Hilbrich, Chief Executive Officer at HALIX.
OTHER NEWS

Download Our
Company Factsheet
your name
your email
organization name