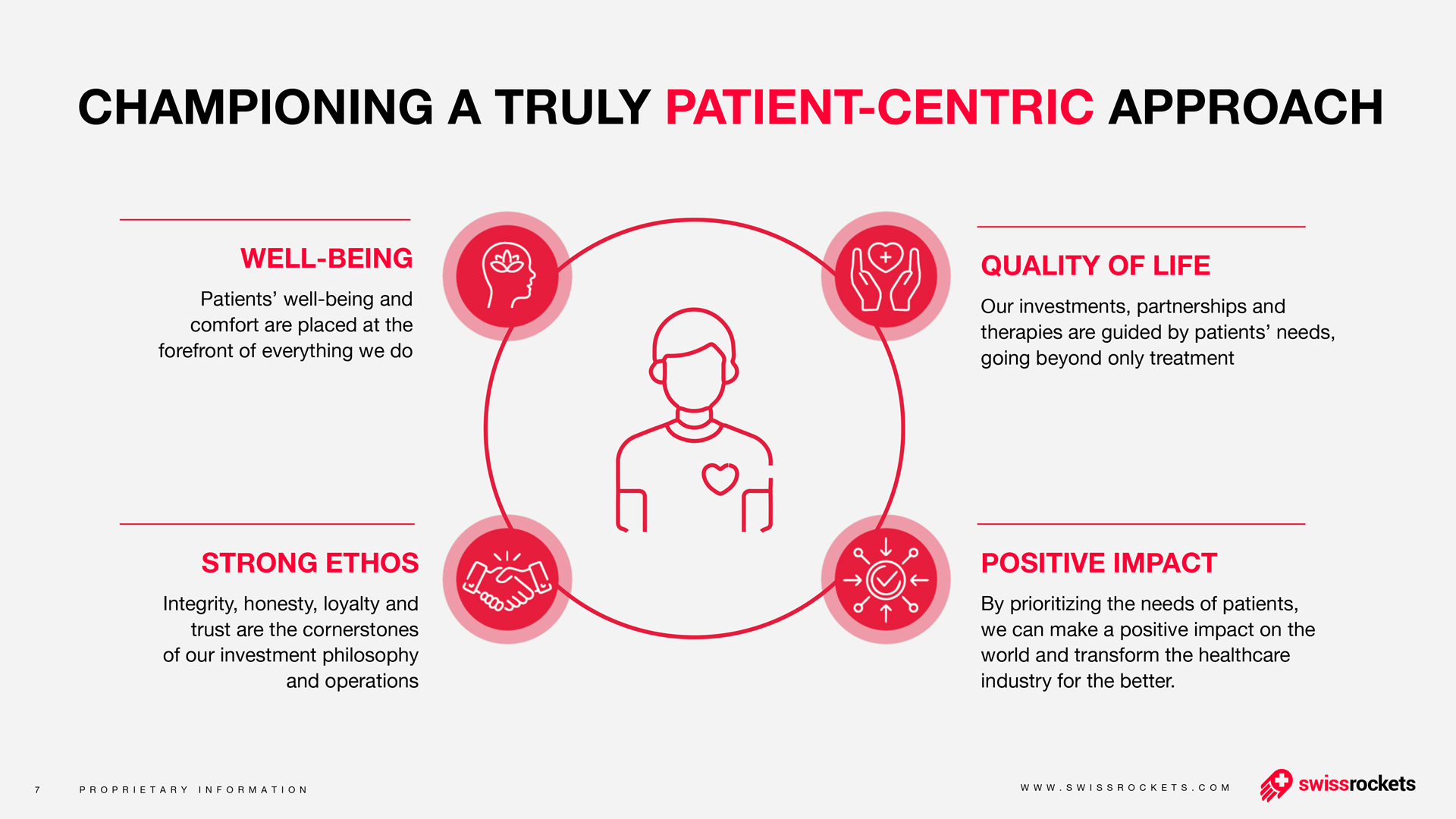
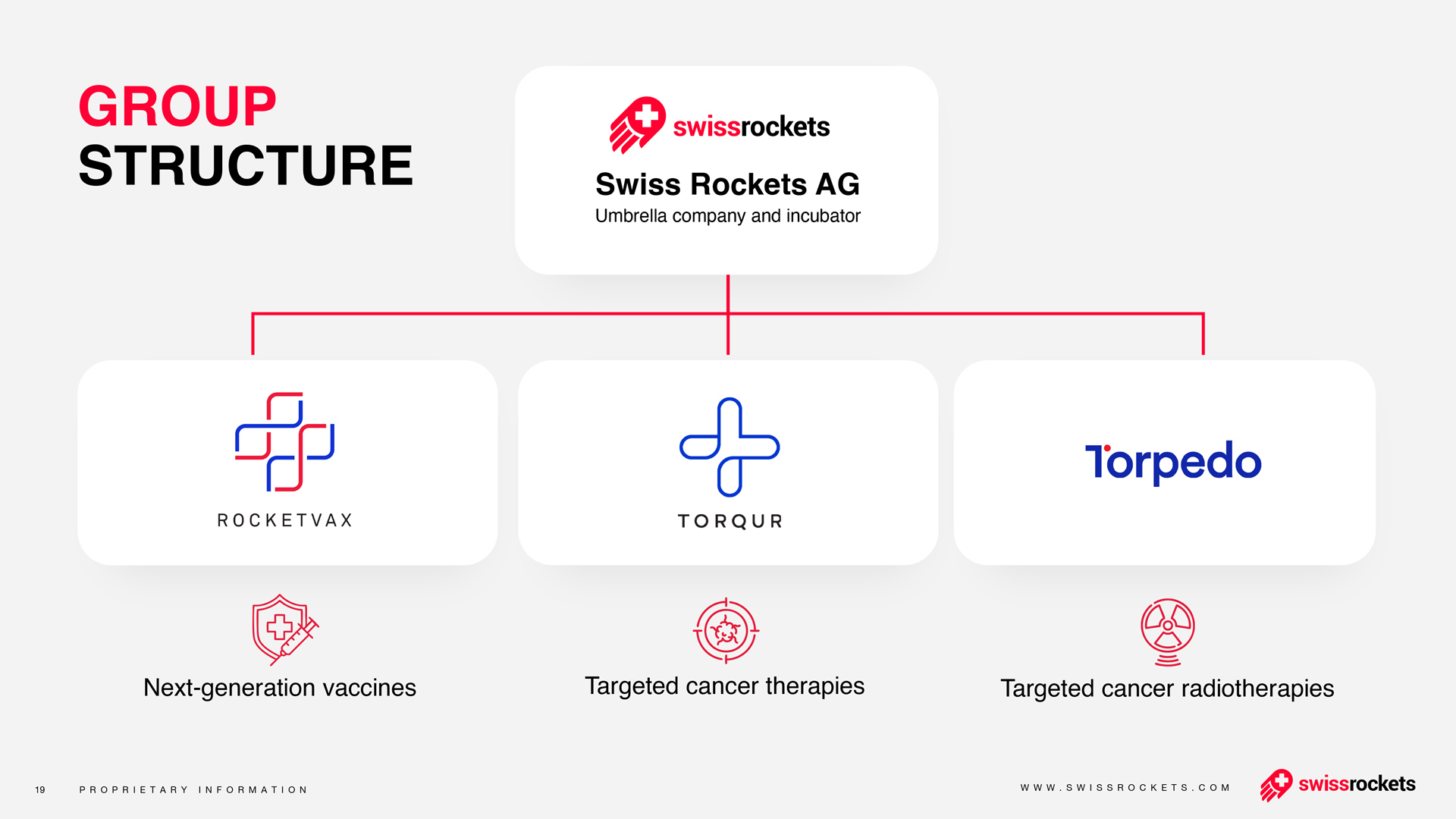
Torqur AG Announces First Patient Dosed in Phase 2 Clinical Trial of Bimiralisib in Actinic Keratosis
The Phase 2 study aims to assess the efficacy and safety of topically administered bimiralisib gel in 40 patients diagnosed with actinic keratosis lesions on the face, scalp, and/or back of hands. This multi-center, randomized, open-label study builds upon promising results from earlier preclinical and Phase 1 studies.
"We are proud to initiate the Phase 2 trial of bimiralisib, marking a further step forward in our mission to develop innovative therapies for actinic keratosis," said Dr. Vladimir Cmiljanovic, Chief Executive Officer of Torqur AG.
"The treatment of the first patient underscores our commitment to advancing novel therapeutic options and addressing the unmet medical needs of individuals affected by actinic keratosis. We eagerly anticipate continuing our assessment of bimiralisib's potential, striving to impact patients' lives significantly, " said Dr. Bruno Osterwalder, Chief Medical Officer.
"As Principal Investigator of this important Phase 2 clinical study, I am honored to lead the research process of bimiralisib. Our team is enthusiastic about the potential of this innovative treatment to address the therapeutic challenges faced by patients with actinic keratosis. This study represents a critical milestone in advancing medical research. We are dedicated to rigorously evaluate the safety and efficacy of bimiralisib to potentially offer a new standard of care for individuals in need." said Prof. Dr. Alexander Navarini, lead Principal Investigator and Chairman of the Department of Dermatology and Allergy at the University Hospital Basel in Switzerland.
Actinic keratosis (AK) is a common pre-cancerous condition that develops on sun-damaged skin, with potential progression to invasive cutaneous squamous cell carcinoma. Current treatments for AK often do not prevent recurrences and present challenges, including local reactions like pain and irritation. PI3K/mTOR signaling has been identified as playing a critical role in AK development, indicating the potential utility of a PI3K/mTOR inhibitor for topical treatment.
Preclinical data demonstrate that topical bimiralisib can potentially prevent or slow down the progression of subclinical AK lesions. Safety results from the first-on-human study indicate that daily administration of topical bimiralisib is well tolerated and safe, suggesting potential advantages over current topical AK treatment options, such as good tolerability, minimal inflammatory triggers, and low risk of infection.
The Phase 2 trial is a collaborative effort involving two renowned dermatology centers in Switzerland, Prof. Alexander Navarini in Basel and Prof. Olivier Gaide in Lausanne. Eligible patients will self-apply topical bimiralisib gel once daily for either 2 or 4 weeks with a potential for a treatment extension. Comprehensive assessments throughout the trial will evaluate the safety profile and therapeutic efficacy of bimiralisib.
For more information about the Phase 2 study of bimiralisib, including enrollment criteria and participating clinical sites, please visit www.clinicaltrials.gov, ID NCT06319794.
OTHER NEWS

Download Our
Company Factsheet
your name
your email
organization name