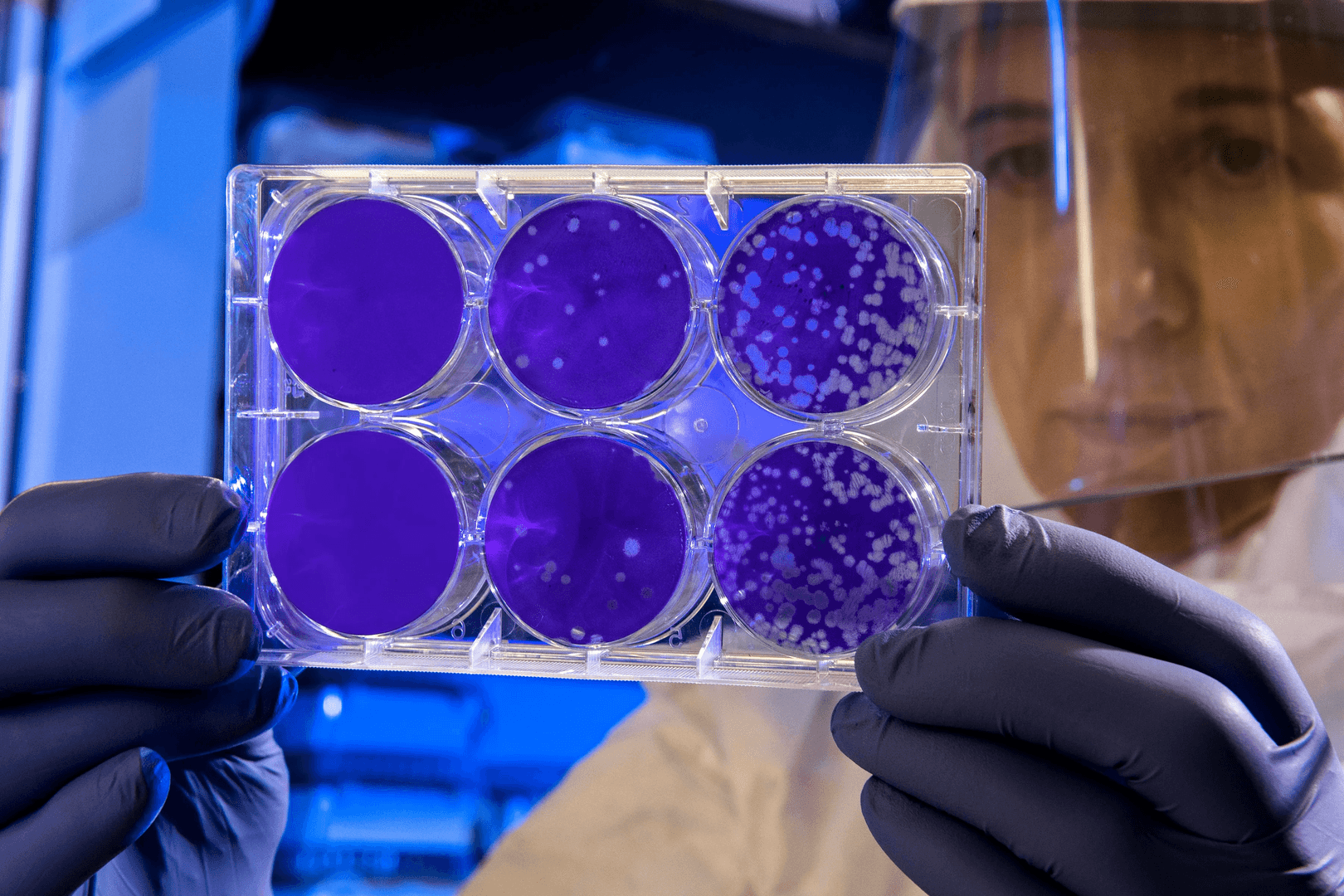

RocketVax AG Announces New Milestones in the Development of Second-Generation COVID-19 Vaccines
RocketVax AG, a subsidiary of Swiss Rockets AG, partnered up with a broadly supported consortium to accelerate the development of second-generation COVID-19 vaccines in March 2022. The consortium is headed by Prof. Volker Thiel, a pioneer in coronavirus research; members of the consortium are leading institutes from Switzerland and Germany, such as the Institute of Virology and Immunology, the University of Bern, the University of Geneva, the Free University of Berlin, and the Friedrich Loeffler Institute.
Two vaccine candidates at an advanced stage
Since the launch of the project as part of the Swiss National Science Foundation’s “COVID-19” National Research Programme (NFP 78), which is supported by the German Research Foundation, experts have succeeded in producing two attenuated SARS-CoV-2 viruses using two different strategies. Thanks to this highly specialised technique, two vaccine candidates will soon be ready for clinical phases I and II. These are the RVX-sCPD9/SARS-CoV-2 (sCPD9) and RVX-OTS/SARS-CoV-2 (OTS) vaccines. The altered sequence (up to 600 mutations) of the attenuated viruses reduces their ability to multiply, so that the immune system can quickly put the viruses under control. This helps generate an optimal immune response. The RVX-sCPD9 vaccine was discovered and further developed at the Free University of Berlin by Dr. Dusan Kunec and Dr. Jakob Trimpert. The RVX-OTS vaccine was discovered and further developed by Prof. Volker Thiel and his team at the Institute of Virology and Immunology and the University of Bern.
With the help of a third technique, another attenuated SARS-CoV-2 virus is also being developed in the Prof. Thomas Klimkait’s research group at the University of Basel, which should also produce a vaccine candidate. This virus is still able to infect cells of the mucosa, but can then no longer reproduce. Such a vaccine has the potential of being used in immunocompromised patients.
The RocketVax vaccines are intended to be administered intranasally and to induce strong mucosal immunity in the upper respiratory tract, offering excellent protection against SARS-CoV-2 infection at the site of virus entry. Most importantly, the RocketVax vaccines showed exceptional results compared to the existing vaccine technologies, be it mRNA-based or adenovirus-based vectors. The RocketVax vaccines demonstrated effective neutralisation of all variants of concern: Beta, Delta, and especially Omicron, which resist neutralisation by mRNA-based or adenovirus-based vaccines. When administered as a booster vaccine after primary mRNA or adenovirus vaccination, the RocketVax vaccines were superior to double mRNA vaccination and double adenovirus vaccination. The animal experiments also showed that animals immunised by the RocketVax vaccine were not reinfected with the natural virus.
(https://www.biorxiv.org/content/10.1101/2022.05.16.492138v1.full.pdf).
Prof. Volker Thiel from the Institute of Virology and Immunology and the University of Bern explains: “Only through intensive cooperation with industry can such a project be realised at this speed. Together, we can test the vaccine candidates in clinical trials and then provide the infrastructure for the production and distribution of the vaccines.”
Start of clinical phases I and II expected in spring 2023
The safety of the attenuated sCPD9 virus has been demonstrated in animal studies and the relevant authorities in the Netherlands and Germany have approved the use of the sCPD9 vaccine candidate in BSL2 (Biosafety Level 2) laboratories. This is important for the production of sCPD9 for the human clinical trials.
For OTS, RocketVax AG has already submitted the application for the work under Biosafety Level 2 in Switzerland, and the applications in the Netherlands, Germany, and the USA will follow soon. The approvals for the start of production of OTS for human clinical trials are pending.
Advantages of the RocketVax AG vaccines
The live vaccines with the attenuated virus can be administered nasally in the form of a nasal spray. They are effective against several SARS-CoV-2 variants and provide mucosal immunity with potentially long-lasting immune protection. This reduces the likelihood of SARS-CoV-2 transmission. The vaccines are suitable as booster vaccines to generate hybrid and broad immunity. The live vaccines are expected to remain stable even at higher temperatures.
Dr Vladimir Cmiljanović, RocketVax CEO, explains: “Furthermore, the data and the results collected during the development of attenuated viruses led to the establishment of a ‵platform technology’. This means that the knowledge can be transferred quickly and easily to combat new viruses.”
About RocketVax AG
RocketVax has its foundation in the ties between Swiss Rockets AG, a Swiss incubator and accelerator for startups with innovative therapies, and a team of expert scientists from the Universities of Basel and Zurich, the ETH Zurich, the University Hospital Basel, the Swiss Tropical and Public Health Institute in Basel, and Gigabases Switzerland AG, a spinoff of ETH Zurich.
RocketVax’s first group of vaccines addresses the SARS-CoV-2 virus and are currently undergoing preclinical testing while preparing for the production of the vaccines for human clinical trials. At RocketVax, proprietary molecular biology technologies are used to develop novel vaccines for infectious diseases like COVID-19, cancer, and auto-immune disorders. Several vaccine candidates in the pipeline are being developed. They include the original live, single-cycle virus vaccine, live-attenuated vaccines against SARS-CoV-2, and a vaccine candidate against cancer.
About Swiss Rockets AG
Founded in 2018, Swiss Rockets AG is implementing a paradigm shift in healthcare. Patients benefit from new therapies developed with innovative and pioneering methods. The Swiss Rockets AG team combines expertise and experience to create innovative medicines focused on cancer and viral diseases.
The founders of Swiss Rockets AG are Dr. Vladimir Cmiljanovic, Dr. Natasa Cmiljanovic, Manuel Ebner, Dr. Thomas Sander and Dr. Thomas Staehelin. Vladimir Cmiljanovic is the CEO, a medicinal chemist, and entrepreneur with more than 15 years of experience in cancer drug development. He is the founder of the Swiss biotech companies PIQUR AG and TargImmune AG. With his sister Dr. Natasa Cmiljanovic, the Chief Scientific Officer of Swiss Rockets AG, he has developed cancer drugs at the University of Basel. He has also founded and managed several biotech companies. Manuel Ebner is a Managing Director at Bank of America Merrill Lynch, Switzerland, and a strategic advisor to Swiss Rockets AG. Dr. Thomas Sander, one of the first employees of Actelion's biotech company, is a scientific advisor to Swiss Rockets AG. Dr. Thomas Staehelin, the co-founder of Swiss Rockets AG, is a member of the Executive Board and Chairman of several shareholder companies and foundations.
Members of the Board of Directors of Swiss Rockets AG are Dr. Vladimir Cmiljanovic (Chairman), Prof. Dr. Michael N. Hall, a renowned researcher and professor at the Center for Molecular Biosciences at the University of Basel, Dr. Natasa Cmiljanovic, a medicinal chemist and clinical scientist with experience in the development of cancer drugs, Dr. Thomas Ladner, a business lawyer, founder and co-founder of several successful start-ups and the World.Minds Foundation, and André Debrunner, a financial expert and fund manager at Northern Trust Switzerland AG.
OTHER NEWS

Download Our
Company Factsheet
your name
your email
organization name