Rocketvax Achieves Major Breakthrough in Live-Attenuated SARS-CoV-2 Vaccine Development
The preclinical data were published on 23 May 2025 in npj Vaccines, published by Nature Portfolio, in an article titled “Live attenuated SARS-CoV-2 vaccine OTS-228 demonstrates efficacy, safety, and stability in preclinical model.”
Key Findings:
- OTS-228 shows excellent safety and high efficacy in Syrian hamster models.
- Maximum dose trials confirm full attenuation and prevention of virus spread.
- Serial in vivo passages reveal no reversion to virulence and high genomic stability.
- Maintains efficacy and attenuation even after 15 in vitro passages.
- Protective dose 50 (PD₅₀) established at <100 TCID₅₀ per hamster in low-dose challenge infection.
The vaccine was scientifically conceived and designed by Professor Dr. Volker Thiel, one of the world’s leading coronavirus researchers:
“OTS-228 is the result of years of coronavirus research and rational vaccine design,” said Professor Thiel. “Live-attenuated vaccines can offer long-lasting and broad immunity, and our data show that OTS-228 has the potential to become a safe and effective tool against current and future SARS-CoV-2 variants.”
The preclinical studies were conducted at the Friedrich-Loeffler-Institut under the direction of Professor Dr. Martin Beer, Director of the Institute of Diagnostic Virology.
Leading the partnership efforts, Dr. Natasa Cmiljanovic, Chief Operating Officer of Rocketvax, added:
“This successful collaboration with the University of Bern and the Friedrich-Loeffler-Institut has been pivotal. The robust preclinical results validate our live-attenuated vaccine platform and position Rocketvax to advance into clinical development. We are committed to making this innovative vaccine available as a global public health solution.”
In addition, Rocketvax has entered a strategic partnership with Emergent BioSolutions, a publicly traded U.S. biotech company, to commercialize Rocketvax vaccines in the U.S. The alliance leverages Emergent’s manufacturing infrastructure and regulatory experience to accelerate worldwide access to Rocketvax’s COVID-19 vaccine platform.Rocketvax is making significant progress in the development of its lead candidate and is preparing for the regulatory submissions to launch first-in-human clinical trials. Discussions with investors, public health agencies, and strategic partners are actively underway to support the next development phase.
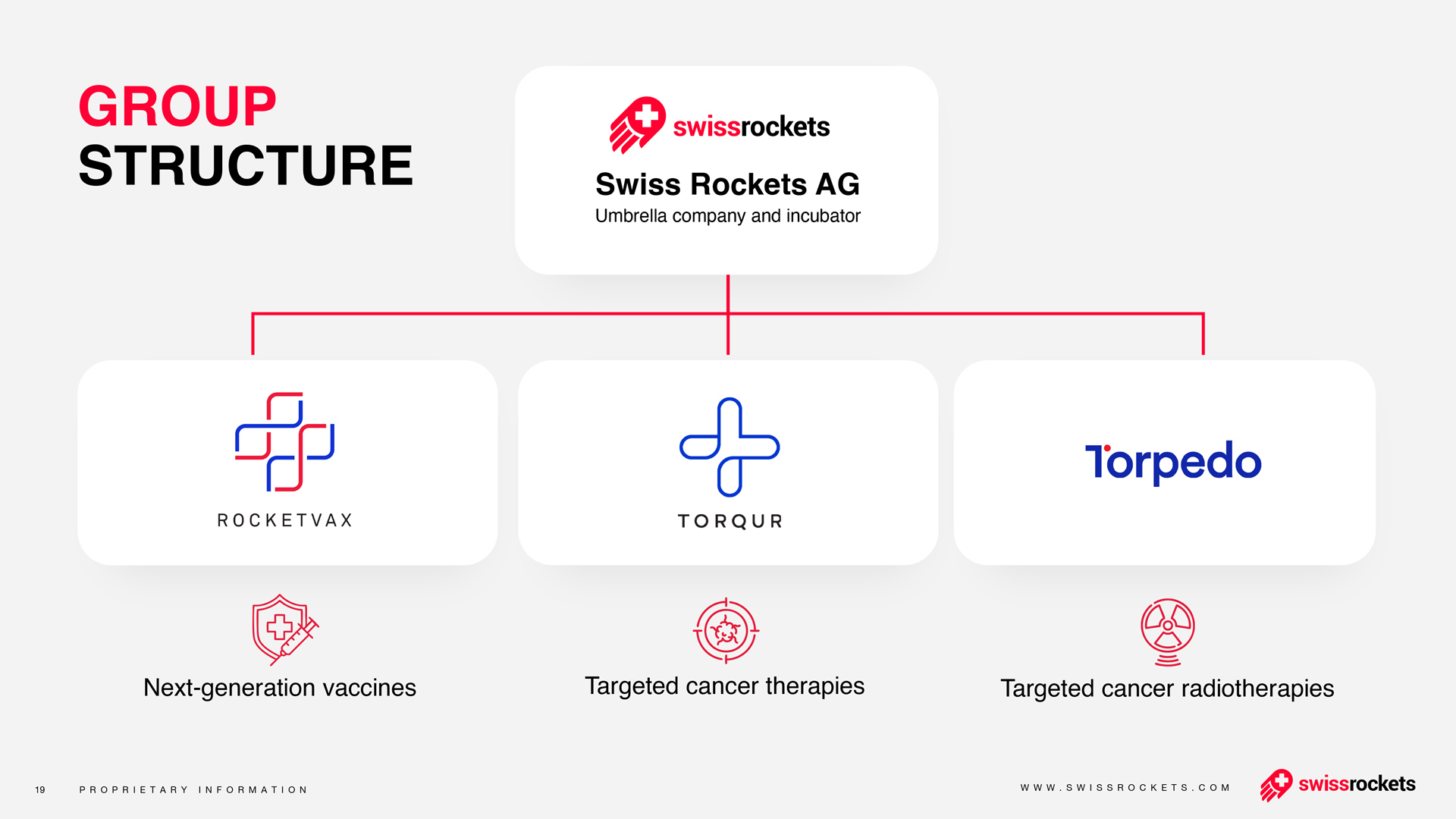
About Swiss Rockets AG
Swiss Rockets AG is an incubator and accelerator supporting the growth of biotech and precision healthcare companies with a focus on oncology. Its portfolio includes radioligand therapies, small molecules, and live-attenuated virus vaccines across preclinical and clinical stages.
OTHER NEWS

Download Our
Company Factsheet
your name
your email
organization name